The decision to undergo Laser-Assisted In Situ Keratomileusis, commonly known as LASIK, marks a pivotal moment for individuals seeking freedom from the daily dependence on corrective eyewear. It is a procedure rightly celebrated for its efficiency and life-altering results in correcting refractive errors such as myopia, hyperopia, and astigmatism. However, the conversation surrounding LASIK must extend beyond its successes to encompass the most common, and often most bothersome, transient side effect: dry eye syndrome. This phenomenon is so prevalent that it should be considered an expected part of the post-operative journey, rather than a rare complication. A thorough understanding of the intricate biological mechanisms that link the surgical procedure to the onset or exacerbation of dry eye is essential for both the surgeon and the patient. This is not simply a matter of temporary irritation; it involves fundamental changes to the ocular surface’s sensory and protective capabilities. We need to move past the superficial discussions and delve into the neuro-anatomical impact, the biomechanical changes, and the subsequent management strategies required to maintain long-term ocular comfort and health after the procedure. The relationship is a delicate equilibrium that is temporarily but profoundly disturbed by the creation of the corneal flap, and appreciating this disturbance is the first step toward successful recovery.
The relationship is a delicate equilibrium that is temporarily but profoundly disturbed by the creation of the corneal flap
The root cause of post-LASIK dry eye is inextricably linked to the neuro-anatomy of the cornea, which possesses one of the highest densities of sensory nerves in the human body. These nerves serve a dual function: transmitting pain and touch signals, and crucially, acting as the primary sensory input that regulates reflex tear secretion and blink rate. The nerves detect the subtle desiccation of the corneal surface and signal the brainstem to prompt an increase in basal and reflex tearing, and to increase the frequency of blinking to redistribute the tear film. When the LASIK flap is created—whether by a microkeratome blade or a femtosecond laser—a significant proportion of these nerve bundles are inevitably severed. This severance occurs circumferentially around the flap, creating an area of central denervation or hypoesthesia. The temporary loss of corneal sensation means that the eye no longer effectively “reports” its state of dryness to the central nervous system. Consequently, the blink rate may drop, and the lacrimal functional unit—the system responsible for tear production—fails to receive the necessary stimulus to produce sufficient tears. This blunted neural feedback loop is the cardinal reason why patients experience profound dryness, often without the corresponding severe foreign body sensation they would normally feel with pre-existing dry eye disease.
The temporary loss of corneal sensation means that the eye no longer effectively reports its state of dryness
The degree of nerve damage is not a fixed variable; it fluctuates based on several critical surgical and anatomical parameters. For instance, the dimensions of the corneal flap are highly influential. A wider-diameter or deeper flap necessitates the severance of a greater number of nerves, which intuitively leads to a more significant and potentially prolonged period of corneal hypoesthesia and subsequent dry eye symptoms. Modern techniques have trended toward smaller flap sizes where feasible to minimize this impact. Furthermore, the regenerative capacity of these nerves varies considerably from person to person. While corneal nerves possess a remarkable ability to regrow, this process is painstakingly slow and often incomplete in the initial months. Full functional re-innervation can take six months, a year, or in some instances, even longer. This protracted healing timeline is why dry eye symptoms, while typically peaking early on, can linger for many months. Patient factors are equally vital. Individuals with pre-existing, subclinical dry eye—such as mild meibomian gland dysfunction (MGD) or low Schirmer scores—are fundamentally operating with a compromised baseline. The surgical insult acts as an acute stressor that pushes a previously manageable, silent condition into a symptomatic, clinically relevant dry eye state. Pre-operative screening must therefore be exceptionally rigorous, extending far beyond a simple questionnaire.
Individuals with pre-existing, subclinical dry eye are fundamentally operating with a compromised baseline
Beyond the neural disruption, the physical alteration of the corneal surface following excimer laser ablation introduces biomechanical factors that compound the tear film instability. The cornea’s new curvature, though visually beneficial, subtly changes the hydrodynamics of tear film flow. Tears, composed of a complex lipid, aqueous, and mucin layer, require a smooth and regular surface to maintain their integrity and uniform distribution. The post-LASIK surface, even when perfectly smooth, can alter the surface tension and the subtle tear meniscus at the flap edge, potentially leading to areas of increased evaporation or tear film break-up. This is a matter of physics interacting with physiology. The reduced aqueous tear production (due to denervation) combines disastrously with the decreased stability (due to altered topography). This double-hit mechanism accelerates the tear film break-up time, meaning the protective layer of tears evaporates much faster than normal between blinks, exposing the underlying corneal epithelium to the drying effects of the air. This leads to the hallmark symptoms of fluctuating vision, gritty sensation, redness, and a paradoxical tearing reflex in severe cases, where the irritated corneal surface triggers an overproduction of low-quality, stress-induced tears.
The post-LASIK surface, even when perfectly smooth, can alter the surface tension and the subtle tear meniscus at the flap edge
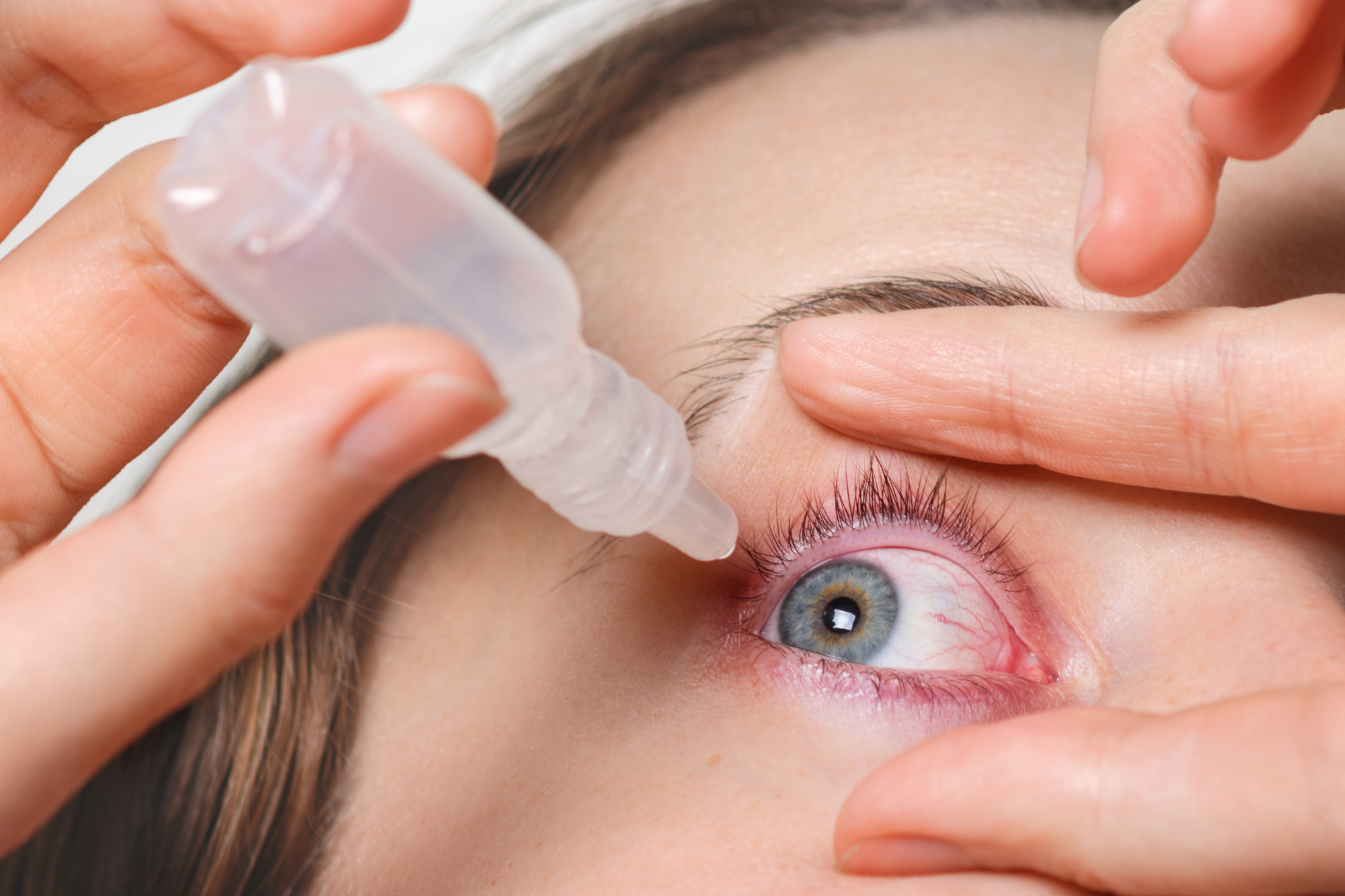
A proactive and patient-centric approach to managing post-LASIK dry eye is mandatory, not optional. The treatment paradigm must be aggressive during the initial recovery phase to support corneal healing and prevent chronic changes. The foundation of therapy rests on the frequent, judicious use of preservative-free artificial tears. Preservatives, designed to inhibit bacterial growth in multi-dose bottles, can be toxic to the already vulnerable and healing corneal epithelium, especially when applied many times a day. Patients must be educated on the correct application frequency, which often needs to be hourly in the first few weeks, regardless of the perceived discomfort level. This proactive lubrication prevents damage and allows the cornea the necessary time to recover its natural function. For many, this is sufficient, and the symptoms gradually resolve within the first three to six months as the nerves regenerate and the ocular surface stabilizes. However, a significant cohort requires more specialized, sustained intervention to bridge the entire recovery gap.
The foundation of therapy rests on the frequent, judicious use of preservative-free artificial tears
When symptoms persist beyond the initial months or are severe from the outset, the focus shifts to anti-inflammatory and tear-sparing agents. Chronic dry eye is fundamentally an inflammatory disease, and the surgical trauma often initiates a period of heightened inflammation on the ocular surface. Prescription topical immunomodulators, primarily cyclosporine (e.g., Restasis, Cequa) or lifitegrast (Xiidra), are utilized to address this underlying inflammation and promote increased basal tear production. These drops are not a quick fix; they require consistent use for several weeks or months to modulate the immune response effectively. Another effective strategy involves preserving the existing tear volume through punctual occlusion. Tiny, biocompatible plugs are placed in the puncta—the small openings that drain tears into the nasal cavity—to physically slow down or block the drainage. This simple, reversible procedure dramatically increases the residence time of natural and artificial tears on the ocular surface, offering significant relief for patients with a predominantly aqueous tear deficiency.
Prescription topical immunomodulators, primarily cyclosporine or lifitegrast, are utilized to address this underlying inflammation
The quality of the tear film is just as important as its quantity, underscoring the necessity of addressing underlying meibomian gland dysfunction (MGD). These glands, located in the eyelids, secrete the essential lipid layer that forms the outermost shield of the tear film, preventing rapid evaporation. If MGD is present—often a pre-existing condition—the thin, aqueous tears produced post-LASIK will evaporate almost instantly, negating the effect of lubrication. Treatment for MGD includes consistent warm compresses and lid hygiene, as well as in-office thermal treatments (e.g., thermal pulsation) to liquefy and express the blocked meibum. Integrating MGD management into the post-LASIK protocol transforms the approach from merely treating symptoms to addressing the multi-factorial pathology of the unstable tear film. The commitment to this holistic treatment approach is often the difference between a satisfied patient with sustained comfort and a frustrated patient whose excellent visual acuity is undermined by persistent ocular discomfort.
Integrating MGD management into the post-LASIK protocol transforms the approach from merely treating symptoms
For the most challenging and non-responsive cases of post-LASIK dry eye, particularly those involving persistent epithelial damage, the use of biologically active tear substitutes becomes necessary. Autologous Serum Tears (ASTs) are derived from the patient’s own blood and contain essential growth factors, epithelial healing nutrients, and vitamins in concentrations that perfectly match the body’s natural tear components. Unlike artificial tears, ASTs actively promote the repair and regeneration of the compromised corneal surface. They provide a high-level, bespoke therapy that bypasses the limitations of commercial products, which lack these critical biological components. Though reserved for severe, treatment-resistant dryness due to the logistical complexity and cost, ASTs represent a powerful therapeutic tool for ensuring the cornea fully recovers its integrity and comfort. This treatment pathway highlights the profound respect for the complexity of the ocular surface required in managing post-refractive surgery sequelae.
Autologous Serum Tears are derived from the patient’s own blood and contain essential growth factors
Effective communication is the linchpin of successful outcomes. Surgeons must manage expectations by emphasizing that some degree of post-operative dry eye is almost inevitable and by thoroughly identifying and risk-stratifying patients during the pre-operative evaluation. A patient who is aware of the potential for a six-to-twelve-month period of consistent eye drop usage is significantly more likely to adhere to the treatment plan and remain satisfied than a patient blindsided by unexpected discomfort. The true measure of success after LASIK is not just the final uncorrected visual acuity, but the long-term, comfortable maintenance of that vision. This requires transparency about the surgical insult to the corneal nerves and the necessary biological healing time. The responsibility falls equally on the patient to commit fully to the prescribed dry eye management protocol, recognizing it as an integral part of the overall surgical success.
Surgeons must manage expectations by emphasizing that some degree of post-operative dry eye is almost inevitable
As refractive surgery technology evolves, the focus on reducing this specific side effect continues to drive innovation. Techniques such as Small Incision Lenticule Extraction (SMILE) are predicated on minimizing the nerve severance by creating a significantly smaller incision compared to the large hinged flap of traditional LASIK. Early clinical evidence suggests that SMILE does indeed result in a lower incidence and faster resolution of dry eye symptoms, offering a glimpse into a future where the trade-off between visual freedom and ocular comfort is further minimized. However, until such technologies are universally applied and proven, the current reality demands an exhaustive, individualized approach to diagnosis and treatment. The connection between LASIK and dry eyes is a direct consequence of surgically interrupting a vital biological pathway, and successful long-term results depend on our ability to aggressively support the eye’s intrinsic, remarkable capacity for healing.